Abstract
Background: Mature T-cell lymphomas (TCL) are an uncommon heterogeneous subset of non-Hodgkin lymphomas for which allogeneic hematopoietic stem cell transplantation (alloHCT) is considered in the relapsed/refractory setting. Outcomes of alloHCT reported through prior registry data has shown that only 31% of patients (pts) with TCL remain disease free 3 years after alloHCT (Smith et al. JCO2013). However, several single institution studies have shown superior outcomes.
Methods: We analyzed the baseline characteristics, treatment history, overall (OS) and progression-free survival (PFS) in consecutive patients with mature TCL who received an alloHCT from 1/1/2000- 5/12/2017 at 6 academic institutions. Patients with the following diagnoses were included: peripheral T-cell lymphoma, NOS (PTCL-NOS); angioimmunoblastic T-cell lymphoma (AITL); anaplastic large cell lymphoma (ALCL), anaplastic lymphoma kinase (ALK) negative; ALCL, ALK positive; ALCL, ALK unknown; adult T-cell leukemia/lymphoma (ATLL); hepatosplenic TCL (HSTCL); extranodal NK/T-cell lymphoma (ENKTL); enteropathy-associated TCL (EATL); cutaneous T-cell lymphoma (CTCL); primary cutaneous ALCL; subcutaneous panniculitis-like TCL (SPTCL); primary cutaneous gamma/delta TCL (GDTCL); blastic plasmacytoid dendritic cell neoplasm (BPDCN).
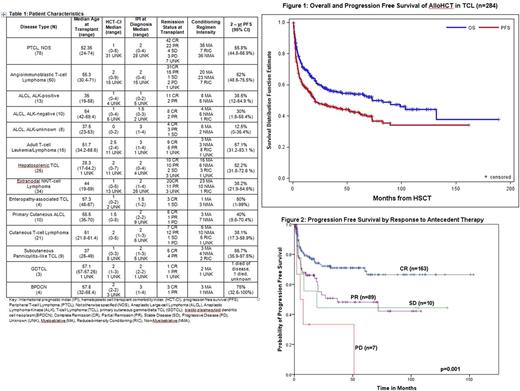
Results: Patient characteristics are shown in Table 1. 284 pts were identified with median age of 50 years (17-74). Of these, 269 had known remission status at the time of transplant. At time of alloHCT, 163 (60.5%) were in complete remission (CR), 89 (33.1%) in partial remission (PR), 10 (3.72%) with stable disease, and 7 (2.60%) with progressive disease. 53 pts received an alloHCT in CR1 and 8 in PR1. 102 pts received an alloHCT in CR2 or beyond and 53 in PR2 or beyond (8 and 28 were in CRx and PRx). 49 pts had a prior autologous transplant. The median hematopoietic cell transplant comorbidity index score was 2 (0-9).
The conditioning regimen was myeloablative in 131, reduced intensity/non-myeloablative in 149 and unknown in 4. Donor type was available for 281 patients: 113 matched related, 106 matched unrelated, 24 mismatched, 13 haploidentical donors, 25 cord blood.
Following alloHCT, 88 pts have progressed to date at a median of 4.65 months (0.72-88.38). With a median follow-up period for survivors of 49.17 months (0.69-186.51), the OS and PFS rate were 60.1% (95% CI: 53.8-65.7%) and 47.8% (95% CI: 41.7-53.7%) at 2 years. (Figure 1) For the disease specific 2-year PFS please see Table 1. 65.1% of the deaths occurred within 12 months of alloHCT. Of 129 patients who have died, 56 died of lymphoma and 30 died of alloHCT complications (death within 30 days of transplant or due to GvHD). 43 pts had an unknown or other cause of death. In this series, there was no significant difference in outcome for patients with AITL, PTCL-NOS, ALK positive ALCL or ALK negative ALCL which carried a median PFS of 52.2 months (95% CI: 17.9-82.9; p=0.247). The rate of transplant related mortality (TRM) at 1 year was 13.2% (95%CI: 8.3-18.1). Of the 281 pts who had data regarding development of graft-versus-host disease (GvHD), 119 (42.3%) developed acute GvHD and 97 (34.5%) developed chronic GvHD. There were no differences in TRM according to recipient age and HCT-CI, (all p-values NS).
Response to prior therapy at the time of alloHCT was associated with PFS (p=0.001). (Figure 2) Median PFS for those with PR, stable disease or progressive disease were 36.8 mo, 19.2 mo, 4.96 mo respectively. In contrast, median PFS was not reached for pts in CR at the time of alloHCT. Degree of donor match was associated with cumulative TRM(p=0.012). For patients who underwent matched related, matched unrelated, or mismatched donor alloHCT, cumulative TRM at 6 months was 2.9% (1.5-7.3%), 7.8%(4.3-14.2%), 14.8%(6.8-32%) respectively. 32 of 88 pts who relapsed survive to date with median survival of 3.55 months post relapse (0.07 -78.6 months).
Conclusions: We present the largest series of alloHCT in TCL. This study suggests that alloHCT is a potentially curative treatment option for patients with TCL. Response to prior therapy was associated with PFS. Mismatched alloHCTs were associated with higher TRM. While 64% of relapses occur within 12 months following transplant, 53.5% of the patients in this series remain alive. This data supports the curative potential of alloHCT in a patient group with otherwise poor survival and limited treatment options.
Mehta-Shah: Celgene: Research Funding; Verastem: Research Funding; Bristol Myers-Squibb: Research Funding. Beaven: GlaxoSmithKline: Other: Spouse's employment; Celgene: Honoraria. Porcu: Galderma: Research Funding; Kura: Research Funding; Kiowa: Research Funding; Celgene: Research Funding; Miragen: Research Funding; Cell Medica: Research Funding; Innate Pharma: Research Funding; Tetralogic: Research Funding. Moskowitz: Seattle Genetics: Honoraria, Research Funding; Incyte: Research Funding; Bristol Myers-Squibb: Consultancy, Research Funding; Takeda: Honoraria; ADC Therapeutics: Research Funding. Horwitz: Celgene: Consultancy, Research Funding; Aileron Therapeutics: Research Funding; Seattle Genetics: Consultancy, Research Funding; Forty-Seven: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Kyowa-Hakka-Kirin: Consultancy, Research Funding; Millenium/Takeda: Consultancy, Research Funding; ADCT Therapeutics: Research Funding; BMS: Consultancy; Mundipharma: Consultancy; HUYA: Consultancy. Jacobsen: GSK: Membership on an entity's Board of Directors or advisory committees; Spectrum: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.